We have developed a powerful and proprietary drug discovery platform called ENIGMAC™ that overcomes the barriers of macrophage drug discovery.
Enabled by unparalleled technology and insight to meet the challenge of understanding the direct effector function of macrophages in disease, ENIGMAC™ has solved the problem of gene editing macrophages: unlocking the opportunity for new target discovery informed by bio-informatics with human genetic data.
ENIGMAC™ DRUG DISCOVERY PLATFORM
ENIGMAC™ enables the translation of human disease-specific macrophage biology into genetically validated and actionable therapeutic targets.
Atlas
Macrophages
Macrophages
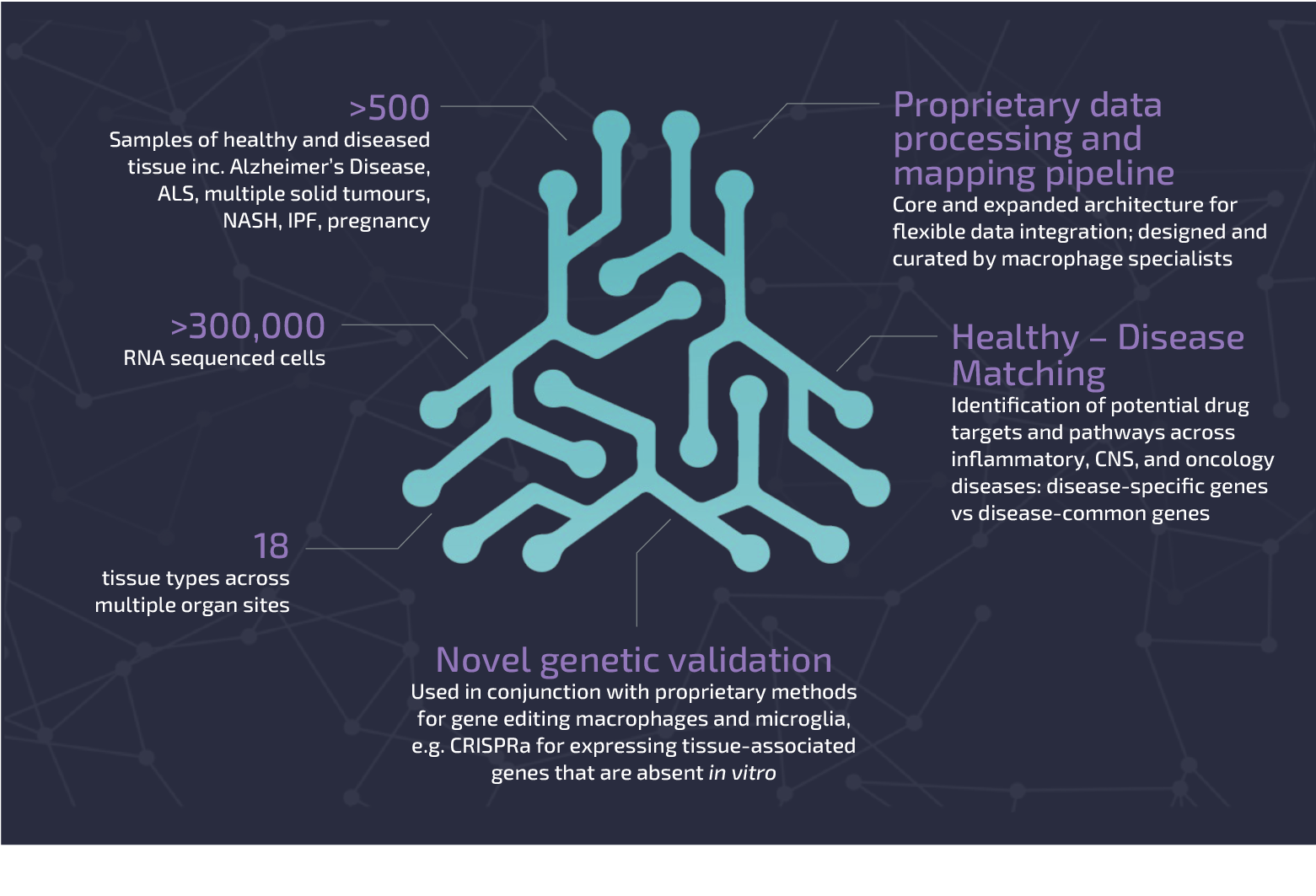
Macrophage Atlas
Our Macrophage Atlas integrates proprietary datasets with meticulously re-annotated public domain data, encompassing both healthy and diseased tissues. The Atlas stands as one of the largest pan-disease and healthy tissue macrophage RNA-seq collections globally. Leveraging this vast resource and our bioinformatics expertise, we can explore fundamental questions about conserved pathways across different disease states—shedding light on the role of macrophage activity and inactivity in driving disease processes.
Gene editing macrophages
The ENIGMAC™ platform enables generation of gene editable macrophages and other cell types. We are able to perform gene knock-up and knock down in genes of interest in IPS derived macrophages in disease relevant models. ENIGMAC™ is used for target identification, validation and drug discovery.
iPSC-derived Macrophages
We use a proprietary human Induced Pluripotent Stem Cell (iPSC) line that yields macrophages and microglia phenotypically and functionally very similar to HUMAN monocyte-derived macrophages (MDM). The iPSC derived macrophages are plastic and can be modelled to different disease states by employing this iPSC system, we can consistently produce millions of macrophages per week, which allows multiple high throughput assays to support target validation and drug discovery.